Monkeypox Still a Public Health Emergency, According to Federal Officials
The epidemic of monkeypox is still a public health emergency, according to the Department of Health and Human Services (HHS).
The public health proclamation was initially released by the HHS in August.
The renewal follows the U.S. Centers for Disease Control and Prevention’s Trusted Source report that over 28,000 instances of monkeypox had been identified in the country, along with eight fatalities.
As soon as the first case of monkeypox was discovered in the United States in May, the number of cases immediately increased, prompting calls for immunizations.
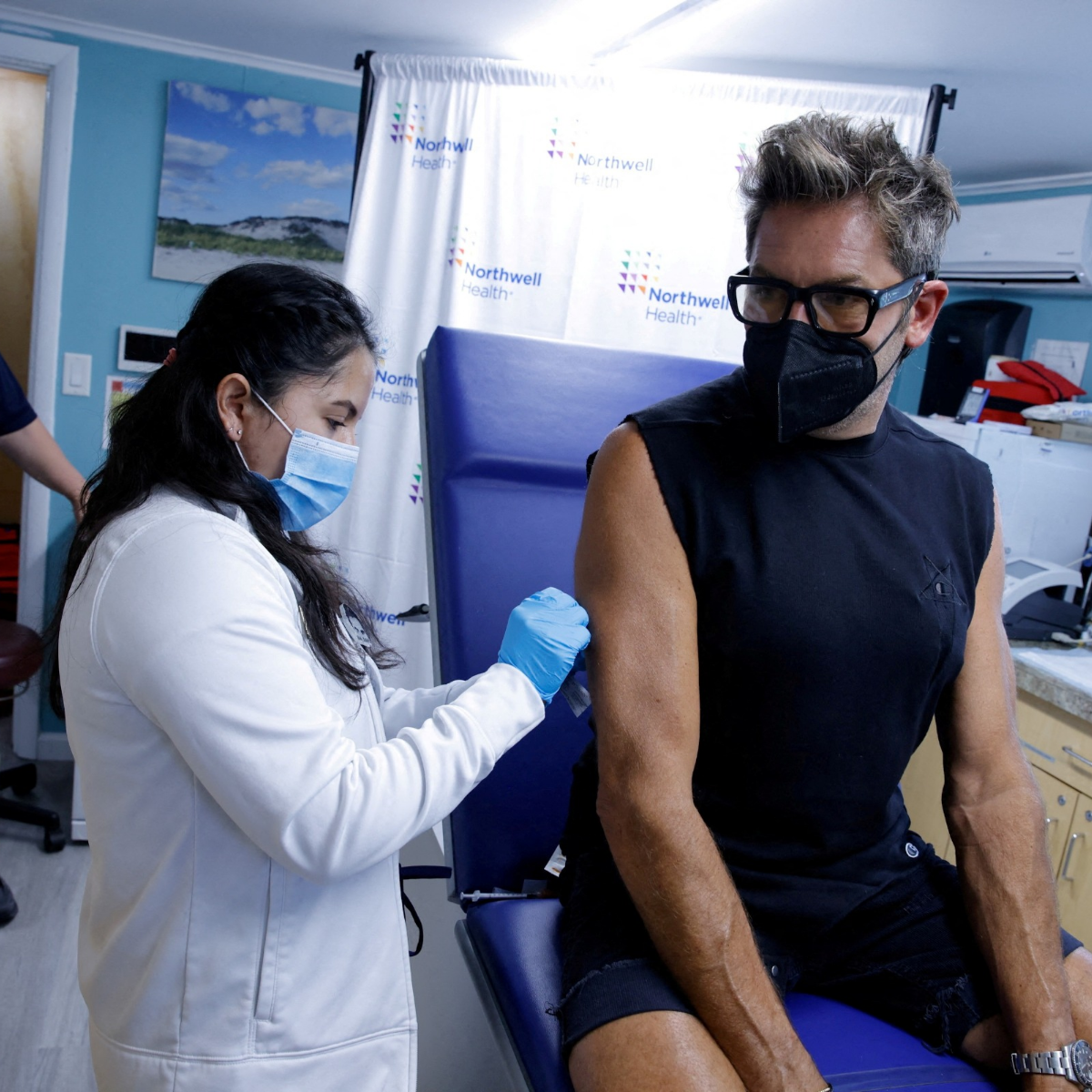
Since being introduced in the summer, more than 1 million vaccinations have been given across the nation. Their implementation, though, hasn’t been entirely simple.
What’s going on with monkeypox vaccines
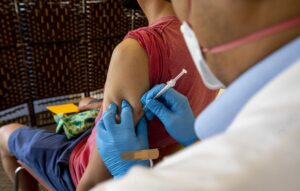
Only a select few people were declared eligible for the shot due to low dose supplies, which sparked a rush for immunisation appointments. This led to worries that the amount of protection would be compromised when the Food and Drug Administration (FDA) authorizedTrusted Source to divide a single dosage into five in order to reach more individuals.
A red, uncomfortable mark is appearing at the injection site for some patients in the meanwhile.
This possibility, according to some publications, is preventing recipients—primarily males who have sex with men—from receiving either their initial or follow-up dose, in part out of fear of stigmatisation, according to The Washington Post.
How monkeypox vaccines are being administered

It’s critical to comprehend how most vaccines are administered before diving into concerns about lesser dosages, regardless of the condition they are intended to prevent. Injectable vaccines come in three different forms: intradermal, subcutaneous, and intramuscular.
Upper arm or leg intramuscular injection locations are frequent. Furthermore, he added, “injecting deeper into the muscle enhances the immune response against the vaccine, optimising protection, for most authorised or fully approved vaccines. It reduces the incidence of local injection site responses as well.
According to Chang, the needle used in subcutaneous injections is “inserted into a layer deeper than the skin, typically the fatty layer between the skin and the muscle.” This method of delivery was initially authorised for the JYNNEOS monkeypox vaccine.
According to Chang, the method “isn’t used as frequently for vaccines because you get more local site injection reactions and the immune response in the skin may not be as robust.”
Dr. Michael Chang, an infectious diseases expert at UTHealth Houston and Memorial Hermann Hospital, told Healthline that the majority of vaccines now on the market, such as the SARS-CoV-2 or influenza vaccines, are intramuscular injections.
Does a lower dose mean weaker protection?
You could believe that you wouldn’t be protected from the illness if you only received 20% of a standard monkeypox vaccine dose.
The research indicates that this isn’t the case, though.
According to Mandavia, the FDA decided to utilise a smaller dose of the vaccine and deliver it intradermally “based on this clinical research including 524 patients.” [The study] showed that] one-fifth of the JYNNEOS vaccination administered [intradermally] into the skin induces a similar immune response as a “full” dosage when administered subcutaneously.
Although the present immunisation undoubtedly provides protection, more research is required to determine the level of defence it provides over an extended period of time. Chang continued, “At this moment, it is uncertain how the one-fifth intradermal dose may affect long-term immunity and protection against monkeypox.”
When to get a follow-up dose
Thankfully, obtaining one-fifth of a vaccination dose does not necessitate four further injections in order to maximise benefits.
“Two doses of the monkeypox vaccination given 28 days apart is considered completely immunised and gives protection,” said Souleles of intradermal delivery of the vaccine.
Your second dose of the immunisation, if you’re under the age of 18, should be given 28 days after the first if you had it subcutaneously.
A New Kind of Monkeypox Testing Is Underway
It can be difficult to test for monkeypox before symptoms occur. Early symptoms may resemble those of other sexually transmitted illnesses, and patients frequently wait until lesions have already appeared before visiting their doctor to get a test (STIs).
The clinical virology laboratory’s medical director at Stanford Health Care is Benjamin Pinsky, MD, PhD. His lab receives requests frequently to examine samples of body fluid for STIs like gonorrhoea and chlamydia.
The monkeypox virus can occasionally be found in samples from patients without lesions when the lab runs these samples through an internal monkeypox test. In contrast to lesion swabs, non-lesion samples have greater false negative results, according to Pinsky. Nevertheless, it might be beneficial to check for other STIs while also looking for cases of monkeypox.